In 2024, Ricoh USA, Inc. (Ricoh 3D for Healthcare) and Kallisio announced a strategic partnership to manufacture and distribute Stentra™ – a 510(k)-cleared, patient-specific oral stent designed to improve radiation therapy for head and neck cancer (HNC) patients.
The Problem
Cancer care is unfortunately widespread globally, and in the United States alone, head and neck cancer therapy is estimated at a cost of $5.46 billion, and $1.58 billion of incremental cost to treat side effects such as radiation-induced oral mucositis.
Whilst radiation plays a crucial role in cancer treatment, it can also pose challenges when it comes to providing precision therapy and protecting surrounding healthy tissue.
In fact, in one study, researchers found that 80% of HNC patients develop radiation-induced complications, such as severe oral mucositis, in the first 3-4 weeks of treatment.
Contrastingly, intraoral stents have been found to reduce severe oral mucositis by 77.6%, which demonstrates the immense need. There were already devices in the market, but they proved to be ill-fitting, ineffective, complicated and cumbersome to use.
The Solution
- 100% case-customised Stentra oral immobilisation device for better patient care
- Rapid, scalable production using RICOH’s 3D printing facilities and post-processing innovations
- Ability to be manufactured on-site at the Point-of-Care

Developed using technology exclusively licensed from MD Anderson Cancer Center, Stentra™ is engineered to protect healthy tissue by precisely displacing and immobilising sensitive areas during treatment, allowing for more targeted and effective radiation delivery.
This innovation is crucial in minimising side effects and enhancing overall treatment outcomes.
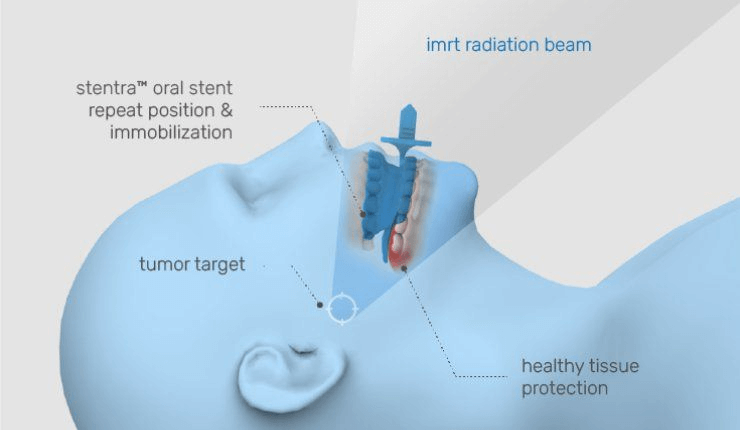
The Results
- Customised, patient-specific oral stents with tongue deviation designed for optimal fit and improved care
- Streamlined workflow enabling delivery from ‘scan to stent’ in as little as three days, requiring just one patient visit
The RICOH-Kallisio partnership helped expedite the final stages of design, production scaling, and market readiness, which took about six months.
Future Scope
Kallisio anticipates rapid growth and is well-positioned to scale production through its partnership with Ricoh 3D for Healthcare – Ricoh 3D’s US counterpart, making Stentra™ readily available to radiation oncology teams.
Ricoh 3D for Healthcare is utilising its network of FDA-registered 3D printing facilities to produce Stentra™, ensuring rapid and efficient distribution to healthcare establishments nationwide, with potential for global expansion…

Could you be interested in supporting this expansion in the UK and European market? Contact us today, or connect with Ricoh 3D’s Medical Lead, Richard Minifie on LinkedIn.
Reflections
“Our collaboration with Kallisio highlights our shared commitment to advancing cancer care by addressing critical needs in toxicity management,” said Gary Turner, Managing Director, Ricoh 3D for Healthcare.
“Stentra™ not only protects the tongue and other healthy tissues from exposure to radiation, but also enhances the precision of radiation therapy, thereby reducing side effects and improving patient quality of life.”
After commercialisation at the end of 2024, radiation oncologists are now able to order Stentra™ through Ricoh 3D for Healthcare’s Clinical Applications Specialists, marking a pivotal step in empowering medical professionals with advanced tools for personalised, patient-focussed care.
Rajan Patel, CEO of Kallisio, stated: “Partnering with RICOH is a natural fit for our mission to transform cancer care. RICOH’s expertise in advanced manufacturing and their robust distribution network, including point-of-care production capabilities and clinical applications team, make them an ideal partner.
“Together, we can ensure that Stentra™ reaches radiation oncology teams efficiently, enhancing treatment precision and patient outcomes on a broad scale.”
Want to learn more about this lifechanging application? Contact the Ricoh 3D for Healthcare team.
Want to discuss application adoption in the UK and European market? Contact us today, or connect with Ricoh 3D’s Medical Lead, Richard Minifie on LinkedIn.